Our offices will be closed for the following New Year National Holidays(including weekends):
- December 27th, 2025 to January 4th, 2026
Thus, incoming emails will be checked intermittently with some possible delays in responding.Thank you for your kind understanding.
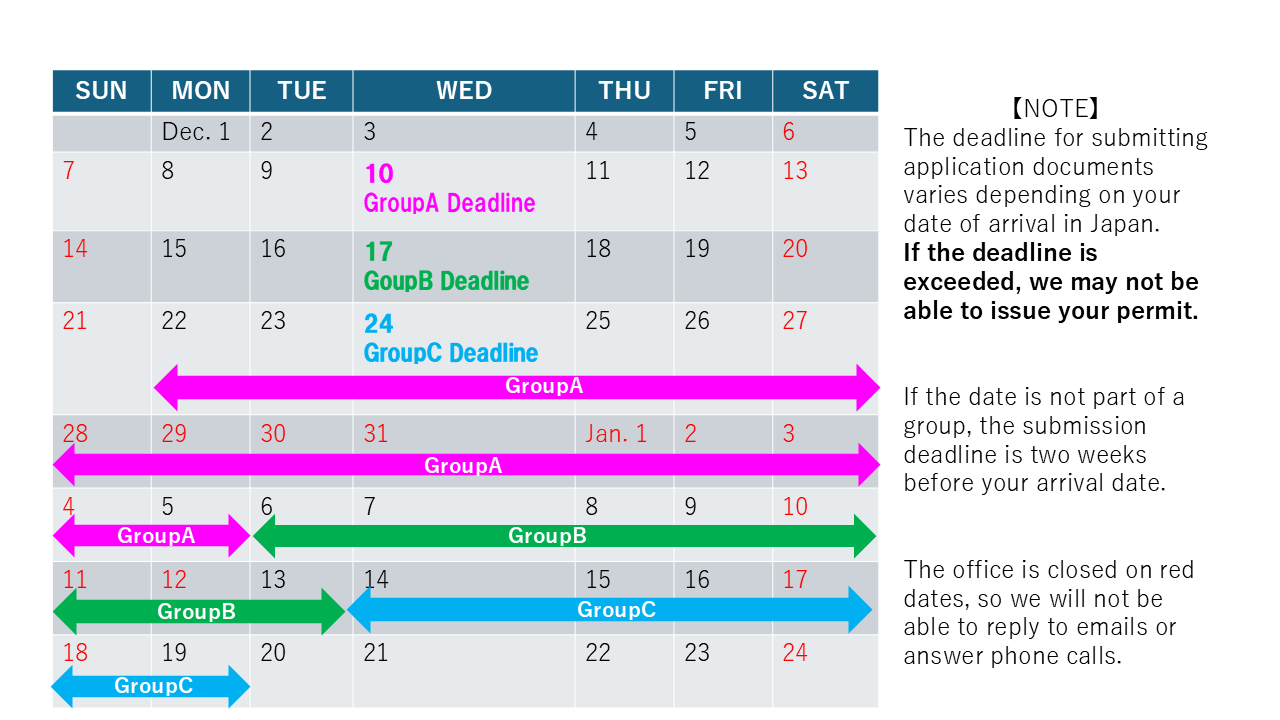
Submitting Timelines for the New Year National Holidays:
- Group A:Entry from 12/22 to 1/5 → DUE DATE 12/10
- Group B:Entry from 1/6 to 1/13 → DUE DATE 12/17
- Group C:Entry from 1/14 to 1/19 → DUE DATE 12/24
- Introduction
- List of Controlled Substances NEW!
- Narcotics / Stimulants' Raw MaterialsNEW!
- Psychotropics
- Prohibited substancesNEW!
- Related Links
- FAQ NEW!
- When you enter/leave Japan carrying your medicine containing controlled substances in Japan for your own medical use, you need to get a permission in advance.
- Procedure depends on the category of the controlled substances; the category is divided into Narcotics, Psychotropics, Stimulants, Stimulants' Raw Materials, Cannabis and Opium.
- Some medicines can't be imported/exported to/from Japan, even if these are prescription medicines in your country.
- First of all, you must check which category your medicine belongs to and go through the corresponding procedure.
- The list does not include brand names of medicines.
- If you are not sure, you should check the category of your medicine with the doctor who prescribed your medicine.
- When you submitted an application form with your electronic signature, please make sure to indicate to that effect in your email.
- In the "Time of entry/departure into/from Japan" section of the application form, please write the date you will enter/leave Japan.
- If you intend to go back to your country with leftover medicines after staying in Japan, please make sure to submit an export application form. If you do not require an export permission, please state so in your email.
- In the "Reason for departure from Japan" section of the application form, please write "To return home" or "To go to another country". If you write "For Vacation", which is a common answer, it will take time to process because we will not know whether you are continuing your vacation in another country or if it is a typo.
- When guardians or parents, are going to fill out application on behalf of underage persons, please fill in the name field of the application form with the name of the person who actually takes the medicine.
In such case, please make sure to describe it as “〇〇’s parent/guardian wrote this on him/her behalf” in the margin of the form followed by their signature. - Your name
- Your current address
- Necessity of medicine for your treatment-the specific reason why you take your medicine, it will not be accertable just "personal use", "medical conditions", "travel"
- A list of your medicine, including doses and the strength
- The signature of the doctor who prescribed your drugs
- The date of issue of medical certificate (Issued date within 3 months)
- If you live in Japan; To the Narcotics Control Department in charge of the area where your domicile is located.
- If you are hospitalized in Japan; To the Narcotics Control Department in charge of the area where the hospital is located.
- If you are entering Japan; To the Narcotics Control Department in charge of the area where you are arriving in Japan.
*If your entry airport and departure airport are different location, please make sure to submit your application forms to the NCD office which covers the entry airport area. - When you enter/leave Japan, you must carry your medicine with yourself.
(You can’t send your medicine to/from Japan and you can’t also entrust carrying it to other people, such as your family.) - Please show the “IMPORT CERTIFICATE/EXPORT CERTIFICATE” to an officer at the Customs.
- Sometimes emails may not come through successfully due to our high security setting or transmission issues, and such failures are not our responsibility. NEW!
- If the attachment is too large, the email may not be delivered.If you are sending a file larger than 10MB, please send it in multiple emails.
And please send a quick follow-up note without any attachments, requesting confirmation from us of the receipt is highly recommended. NEW! - Your name
- Name of your disease
- Necessity of medicine for your treatment
- A list of your medicine, including doses and the strength
- The signature of the doctor who prescribed your medicines
- The date of issue
- Heroin
- Opium powder
- Methamphetamine and Amphetamine(INN:Dexamphetamine, Levamfetamine)
- Methaqualone
- For foreigners and travelers (Ministry of Health, Labour and Welfare)
- Information for those who are bringing medicines for personal use into Japan
- Can I bring ADDERALL (*approved in the US, for ADHD treatment) into Japan?
- No.
Amphetamine, Active Pharmaceutical Ingredient (API) of ADDERALL, is controlled as “Stimulants” under the Stimulants Control Act, and cannot be imported into Japan, even for treatment purposes. - Can I bring VYVANSE (*approved in the US, for ADHD treatment, also authorized in other countries named as ELVANSE, VENVANSE, ADUVANZ, TYVENSE, etc.) into Japan?
- Yes.
Lisdexamfetamine, API of VYVANSE, is controlled as "Stimulants' Raw Materials" under the Stimulants Control Act.
You can import/export it into/from Japan, by getting an advanced permission.
Please click here.
To accommodate the above schedule, if you are coming to Japan during December 22nd through January 5th, please send us complete application documents by December 10th.
To accommodate the above schedule, if you are coming to Japan during January 6th through January 13th, please send us complete application documents by 17th December.
To accommodate the above schedule, if you are coming to Japan during 14th January through January 19th, please send us complete application documents by December 24th.
Contents
on carrying medicine containing controlled substances for travelers entering or leaving Japan.
Introduction
List of Controlled Substances
Controlled Substances List in Japan. NEW!
Narcotics/Stimulants' Raw Materials
If your medicine is in “Narcotics” and/or “Stimulants’ Raw Materials”, you need to get a permission before you enter/leave Japan.
【Reference】
Narcotics:Morphine, Fentanyl, Oxycodone, Codeine, Tapentadol...
Srimulants Raw Materisls:Lisdexamfetamine(Vyvanse, Elvanse...), Pseudoephedrine...
Psycotropics:Methylphenidate, Zolpidem...
General medicines (unregulated):Tramadol...
Required Documents for Application
You need to submit the following documents.
1. Application form (IMPORT)(*for entering Japan)
2. Application form (EXPORT)(*for leaving Japan)
【NOTE】
3.Medical certificateNEW!
You need to get a medical certificate from the doctor who prescribed your medicine. The medical certificate must include:
【NOTE】
When the doctors signed with their electronic signature on a medical certificate, please make sure to indicate to that effect in the medical certificate or your e-mail.
4.Photos of the package of your medicine or relevant documents of the medicine
We need accurate information on your medicine, especially on name and strength of it.
【NOTE】
Our email system cannot view "HEIC files". Please send your photos as "JPEG files", "PDF files" or "Word files".
Application Procedure
By e-mail / FAX / mail, submit above documents to:
【NOTE】
You should apply at least 14 days prior to your travel. If there are not 14days until your arrival date, please contact us.
Please note that contacting us does not necessarily mean that your application will be handled.
Note
Psychotropics
Procedures for Import / Export of psychotropics by carrying
1) The psychotropics listed in the following Table(*3) can be imported / exported. If you intend to import / export the psychotropics equal to or less than the amount indicated in the Table(*3) (excluding injection form), you don't need a certificate written by your doctor nor the permission by authorities under the "Narcotics and Psychotropics Control Law".
2) If you intend to import / export the psychotropics more than the amount indicated in the Table(*3) or those in injection form, you should have a certificate(*2) written by your doctor identifying the disease, the necessity of the drug (psychotropics) for your treatment, the names of psychotrpics and their quantities prescribed.
*1. If you import the psychotropics more than 1 month supply, please contact the following E-mail adress.
*2. Certificate should be written by the doctor who prescribed your medicine, and must include;
*3. The Table is here.
Prohibited Substances NEW!
No individual travelers can import/export medicines including the following substances, even if they are prescribed medicines in your country;